You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Evaporust - first time user / review
- Thread starter JOEPRO
- Start date
Sergei Slovenija
Member
- Messages
- 1,267
- Location
- Slovenija Ljubljana
Information is disclosed by law about the content of chemicals in the product that have a CAS number.What does this mean then? Is that the exact formula of evaporust(?) Is it possible to produce some at home then...
Sergei Slovenija
Member
- Messages
- 1,267
- Location
- Slovenija Ljubljana
I don't see any form of phosphoric acid. Sodium salt of sulfonic acid is. It is a very strong acid and very interesting. When I have time, I’ll order these reagents and try it. The recipe is not as simple as it seems, IMHO.Is that water, phosphoric acid (in some form) and an emulsifier?
Sergei Slovenija
Member
- Messages
- 1,267
- Location
- Slovenija Ljubljana
Sulphamic Acid (Sulfamic) - Descaler / Rust Remover

Buy Sulphamic Acid Descaler, Metal Cleaner & Rust remover UK - Boilers
Sulphamic acid - Powerful Descaler, Rust & Limescale Remover. Excellent cleaner for Toilets, grouting, boilers, metal, ceramics. Dissolves efflorescence and other mineral deposits. 100g to 25kg packs. Prices include delivery
mistralni.co.uk
That was my thought on the ingredients as well - not phosphoric acid!Sulphamic Acid (Sulfamic) - Descaler / Rust Remover

Buy Sulphamic Acid Descaler, Metal Cleaner & Rust remover UK - Boilers
Sulphamic acid - Powerful Descaler, Rust & Limescale Remover. Excellent cleaner for Toilets, grouting, boilers, metal, ceramics. Dissolves efflorescence and other mineral deposits. 100g to 25kg packs. Prices include deliverymistralni.co.uk
Sounds like it will do the job!
Combined with a surfactant of course.

Sulphamic Acid/ Sulfamic Acid | Bonnymans
LARGER SIZES AVAILABLE ON REQUEST - PLEASE CONTACT FOR PRICING Sulfamic acid, also known as amidosulfonic acid, amidosulfuric acid, aminosulfonic acid, and sulfamidic acid, is a molecular compound with the formula H3NSO3. This colorless, water-soluble compound finds many applications. CAS No...
 www.bonnymans.co.uk
www.bonnymans.co.uk
Has many uses - denture cleaning tables and fire extinguishers amongst many others!
Looks like it might do the job though.
Seems to be the cheapest for smaller amounts - just bought 500gm to try.
rtcosic
Forum Supporter
- Messages
- 2,969
- Location
- Warwickshire
Seems to be the cheapest for smaller amounts - just bought 500gm to try.
The CAS no of Sulphamic Acid is 5329-14-6
So whilst it can be used as a derusting agent it isn't a decalred ingredient of Evvporust.
Sergei Slovenija
Member
- Messages
- 1,267
- Location
- Slovenija Ljubljana
I did some digging on Google. He banned me from the patent search, there are too many requests, you see, ahah)))The CAS no of Sulphamic Acid is 5329-14-6
So whilst it can be used as a derusting agent it isn't a decalred ingredient of Evvporust.
Sulfamic and methanosulfamic acid are not part of evaporasts, but their derivatives, and salts of these acids, work very interestingly on metals and their oxides and hydroxides.
The ones listed in the German MSDS “Just fertilizer and just surfactants” work a little differently.
I dug up an old secret Soviet textbook on petrochemistry and rolled a couple of high-rolled cigarettes from its pages. We need to think about it in our spare time.
I remember back in the late 70s or early 80s buying a product called Rust X bio. It said it was ph neutral and totally safe to use.
The product was a luminous green jelly. Worked amazingly well. Wash off afterwards.
Tried it on my air rifle barrel and it sadly removed all the bluing. Was amazing on tools though
The product was a luminous green jelly. Worked amazingly well. Wash off afterwards.
Tried it on my air rifle barrel and it sadly removed all the bluing. Was amazing on tools though

Sergei Slovenija
Member
- Messages
- 1,267
- Location
- Slovenija Ljubljana
It was St. Greta's jellyI remember back in the late 70s or early 80s buying a product called Rust X bio. It said it was ph neutral and totally safe to use.
The product was a luminous green jelly. Worked amazingly well. Wash off afterwards.
Tried it on my air rifle barrel and it sadly removed all the bluing. Was amazing on tools though
It was St. Greta's jelly

Ross365
Member
- Messages
- 2,047
- Location
- UK
That MDS shows that there is phosphate in some form in Evaporust and I wasn't expecting that. Presumably it means that after treatment with Evaporust you have metal phosphate compounds on the surface of what you are working on and you may not want that. Is the paint you are going to use intened for use on a phosphated surface  . On the other hand, citric acid does not leave anything on the surface after use.
. On the other hand, citric acid does not leave anything on the surface after use.
 . On the other hand, citric acid does not leave anything on the surface after use.
. On the other hand, citric acid does not leave anything on the surface after use.Sergei Slovenija
Member
- Messages
- 1,267
- Location
- Slovenija Ljubljana
The chemical reaction that occurs when we add (NH4)3PO4 to H2O is a simple dissolution or dissociation reaction. In this reaction, (NH4)3PO4, which is a solid compound, dissolves in water to form ions.That MDS shows that there is phosphate in some form in Evaporust and I wasn't expecting that. Presumably it means that after treatment with Evaporust you have metal phosphate compounds on the surface of what you are working on and you may not want that. Is the paint you are going to use intened for use on a phosphated surface. On the other hand, citric acid does not leave anything on the surface after use.
The equation for this reaction can be written as:
(NH4)3PO4 + H2O -> NH4+ + NH4+ + NH4+ + PO4^3- + H2O
In this equation, (NH4)3PO4 is the solid compound and H2O is water. When (NH4)3PO4 is added to water, it dissociates into individual ions. Each (NH4)3PO4 molecule breaks apart to form three NH4+ ions (ammonium ions) and one PO4^3- ion (phosphate ion). Additionally, the water molecule remains intact.
Overall, the reaction involves the dissolution of (NH4)3PO4 in water, resulting in the formation of ammonium ions (NH4+) and phosphate ions (PO4^3-).
This is an oxidation-reduction (redox) reaction:

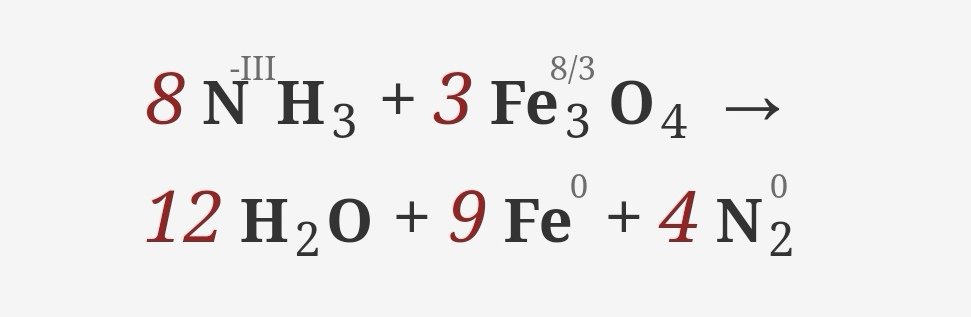
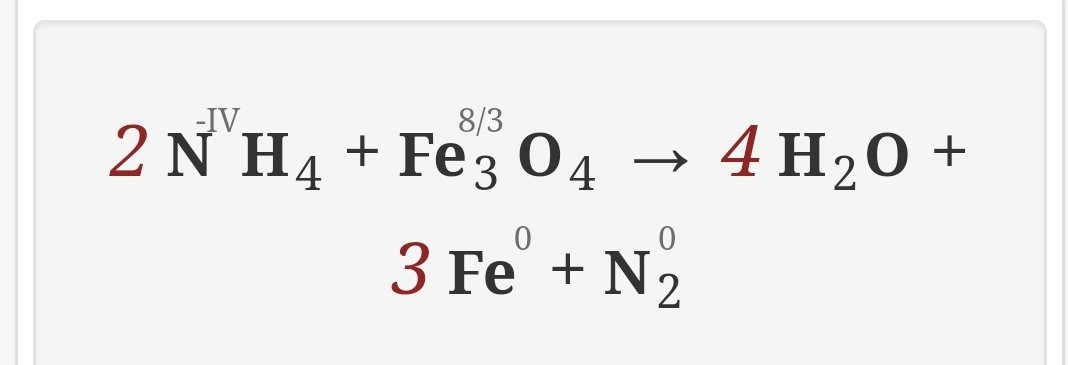
Ross365
Member
- Messages
- 2,047
- Location
- UK
The chemical reaction that occurs when we add (NH4)3PO4 to H2O is a simple dissolution or dissociation reaction. In this reaction, (NH4)3PO4, which is a solid compound, dissolves in water to form ions.
The equation for this reaction can be written as:
(NH4)3PO4 + H2O -> NH4+ + NH4+ + NH4+ + PO4^3- + H2O
In this equation, (NH4)3PO4 is the solid compound and H2O is water. When (NH4)3PO4 is added to water, it dissociates into individual ions. Each (NH4)3PO4 molecule breaks apart to form three NH4+ ions (ammonium ions) and one PO4^3- ion (phosphate ion). Additionally, the water molecule remains intact.
Overall, the reaction involves the dissolution of (NH4)3PO4 in water, resulting in the formation of ammonium ions (NH4+) and phosphate ions (PO4^3-).
This is an oxidation-reduction (redox) reaction:
View attachment 425083 View attachment 425084 View attachment 425085
And what happens to the phosphate ions? In the water, the phosphate ions are effectively forming phosphoric acid. I would expect that they would react with the surface of the steel to form an insoluble layer of metal phosphate.
Sergei Slovenija
Member
- Messages
- 1,267
- Location
- Slovenija Ljubljana
There is also a second component, the sodium petroleum salt of sulfonic acid (a strong acid, like sulfuric acid) + its dissociation. I noticed the formation of free iron ions recovered from rust; it is easier to react with them than with a monolithic iron alloy. Interesting reactions occur in this compote, but the school chemistry course ended too long ago.And what happens to the phosphate ions? In the water, the phosphate ions are effectively forming phosphoric acid. I would expect that they would react with the surface of the steel to form an insoluble layer of metal phosphate.

PS - The evaporust solution depletes over time. I'm not going to buy it as an experiment, but it may be possible to freshen it up by adding Diammonium phosphate or phosphoric acid.
Sergei Slovenija
Member
- Messages
- 1,267
- Location
- Slovenija Ljubljana
So, sodium petroleum sulfonate (as a surfactant), as I understand it, forms micelles and displaces water from the metal, competing with proton exchange, cations and anions.
The question is what reacts with what in a chemical reaction, and with what micelles are formed, with iron ions, with iron phosphorus salts. Further, sodium petroleum sulfonate, as I understand it, comes in different origins (artificial and natural from petroleum products), and different molecular weights. It’s still a big question whether liquid (brown oily sodium petroleum sulfonate) is used in the evaporust formula, or whether pure sodium sulfonate (powder) is mixed in there.
PS - The CAS number corresponds to the liquid, but a mixture of powder and liquid may mask the full formulation. Well, this is from the realm of conspiracy theory (assumption), I’m not sure that such a practice exists anywhere in developed countries and harsh packs of wolf-lawyers.
The question is what reacts with what in a chemical reaction, and with what micelles are formed, with iron ions, with iron phosphorus salts. Further, sodium petroleum sulfonate, as I understand it, comes in different origins (artificial and natural from petroleum products), and different molecular weights. It’s still a big question whether liquid (brown oily sodium petroleum sulfonate) is used in the evaporust formula, or whether pure sodium sulfonate (powder) is mixed in there.
PS - The CAS number corresponds to the liquid, but a mixture of powder and liquid may mask the full formulation. Well, this is from the realm of conspiracy theory (assumption), I’m not sure that such a practice exists anywhere in developed countries and harsh packs of wolf-lawyers.
Ross365
Member
- Messages
- 2,047
- Location
- UK
It appears to be a more complex product than many of us may have thought; I've not used it myself but I'm aware that others are consistently reporting that it works very well in terms of rust removal.
As for the issue of phosphate surface layers, I do think this is a significant question. The best illustration might be if someone was going to use one of the zinc loaded primers; good electrical conductivity between the zinc particles in the paint and the substrate metal is essential to get the best out of these paints. I suspect the same may be true for zinc phosphate primers.
I have used phosphating agents in the past, (I had good results when using simple phosphoric acid and enamel paints) but haven't used them for ~20 years. I have had some poor outcomes with some commercial phosphating agents
As for the issue of phosphate surface layers, I do think this is a significant question. The best illustration might be if someone was going to use one of the zinc loaded primers; good electrical conductivity between the zinc particles in the paint and the substrate metal is essential to get the best out of these paints. I suspect the same may be true for zinc phosphate primers.
I have used phosphating agents in the past, (I had good results when using simple phosphoric acid and enamel paints) but haven't used them for ~20 years. I have had some poor outcomes with some commercial phosphating agents
Sergei Slovenija
Member
- Messages
- 1,267
- Location
- Slovenija Ljubljana
An example from my practice: almost a month ago, I pickled an angle profile made of low-carbon, hot-rolled steel in a solution of citric acid + EDTA + methenamine. It’s been a lot of rain and high humidity, but the steel doesn’t want to rust and in some places it’s still white. And I need to get a layer of uniform rust for tannin treatment. Wet rags didn't fix the situation. What was it? no phosphates or zinc. I was going to build a box for boiling water + a fan to create a hot and humid atmosphere. The rapid rust process will be useful to me, including for “rusty bluing”, but I will deal with it later.It appears to be a more complex product than many of us may have thought; I've not used it myself but I'm aware that others are consistently reporting that it works very well in terms of rust removal.
As for the issue of phosphate surface layers, I do think this is a significant question. The best illustration might be if someone was going to use one of the zinc loaded primers; good electrical conductivity between the zinc particles in the paint and the substrate metal is essential to get the best out of these paints. I suspect the same may be true for zinc phosphate primers.
I have used phosphating agents in the past, (I had good results when using simple phosphoric acid and enamel paints) but haven't used them for ~20 years. I have had some poor outcomes with some commercial phosphating agents